DRAFT Sept 2, 2021 Letter to FDA Commissioner Janet Woodcock regarding FDA Approval
I was asked to draft a letter to Janet Woodcock regarding the FDA approval of COMIRNATY for a Senator. This is the draft letter that I sent on September 2, 2021.
September 2, 2021
Janet Woodcock, M.D
Acting Commissioner
Food and Drug Administration
10903 New Hampshire Ave
Silver Spring, MD 20993
Dear Dr. Woodcock:
On August 22, 2021, I sent you a letter urging you to reconsider the Food and Drug Administration’s (FDA) decision to forgo assembling a Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting before approving a biologic license application (BLA) for the EUA Pfizer-BioNTech mRNA COVID-19 ‘vaccine.’
Your August 23, 2021, written response, referred to herein as “FDA BLA RESPONSE”, did not reasonably address concerns regarding the effectiveness, safety risks, and serious adverse events (SAEs) of the of the EUA Pfizer-BioNTech COVID-19 Vaccine. Following are a few examples of your lack of transparency, credibility, and integrity in your FDA BLA RESPONSE.
FDA BLA RESPONSE: ¶1:2
“This decision was reached after careful review and consideration, noting that there was no new substantive concerns regarding the safety or efficacy of this Pfizer-BioNTech vaccine since the prior two advisory meetings at which it was discussed.” – Janet Woodcock, MD
FDA CLAIMS “NO NEW SUBSTANTIVE CONCERNS REGARDING SAFETY OR EFFICACY OF THE EUA PFIZER-BIONTECH COVD-19 VACCINE”
Prior to your August 23rd written response and after the most recent VRBPAC meeting was held on June 10, 2021, on August 9, 2021, the Mayo Clinic published a well-designed, retrospective analysis of 636,053 patients, 16 years of age or older, across 5 states, of subjects who ALL received PCR tests between December 1, 2020 and July 19, 2021. Subjects received either the EUA Pfizer-BioNTech ‘vaccine’ (BNT161b), the EUA Moderna ‘vaccine’ (mRNA-1273), or no medical intervention at all.
The investigators did a meticulous job at exact matching the members of the triple cohort study groups by sex, race, ethnicity, state of residence, and frequency of PCR test history.
The Mayo Clinic Study Objectives were to determine the following outcomes of vaccine effectiveness (VE) and vaccine efficacy for each cohort:
Vaccine Efficacy (VE) = reduction in IRR SARS-CoV-2 Infection* (+PCR)
· *VE is the primary outcome of all the COVID-19 Vaccines
· See page 15, ¶11, Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines
Vaccine Effectiveness Outcomes
· COVID-19 associated Hospitalization - within 21 days of infection = (+PCR)
· COVID-19 associated ICU Admission - within 21 days of infection = (+PCR)
· COVID-19 associated Mortality - within 28 days of infection = (+PCR)
· Breakthrough Infection = +PCR confirming SARS-CoV-2 infection occurring at least 14 days after the second dose of mRNA1273 or BNT162
The Mayo Clinic Study investigators provided specific details on the Minnesota Cohort yielding 3 cohorts of 25,689 subjects each.
VACCINE EFFICACY
Regarding “vaccine efficacy” (VE), both the Pfizer-BioNTech (BNT162b) and Moderna (mRNA-1273) ‘vaccines’ were able to reduce the rate of infection (IRR) by about 1% each as compared to no medical intervention at all. Although the Mayo Clinic data shows that Moderna was initially 86% more successful than placebo in reducing risk of infection by 1% as compared to Pfizer’s 76% ‘vaccine efficacy’ neither of these data points have any meaningful clinical benefit in preventing infection from or the spread of the SARS-CoV-2 virus. Over a period of a few months, Moderna’s ‘success rate at doing nothing of clinical benefit’ waned to 76% and Pfizer’s to 42%.
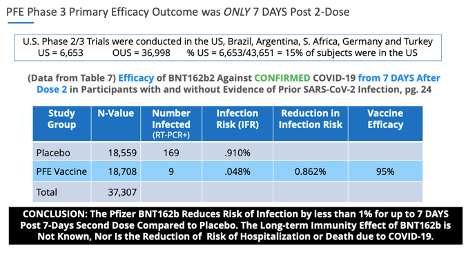
The VE data points were similar in Pfizer’s Phase 3 trials. Pfizer’s primary endpoint outcome was 95% ‘vaccine efficacy’ in reducing the risk of infection by less than 1% (0.86%) at 7 days post second dose.
Dr. Woodcock, you have decades of experience in determining whether a drug or vaccine proves to deliver efficacy of ‘clinical significance” not just “statistical significance,” as well as the importance of communicating clear and accurate safety and efficacy claims to the American people under 21 USC Sec. 502 – False or Misleading claims.
Per page 16, ¶1, Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines, the risk of rate of infection for SARS-CoV-2 is 1.3%.
Vaccine Efficacy (VE) BENEFITS - QUESTIONS:
1. In your expert opinion, do you believe that a 95% chance in reducing the risk of the rate of SARS-CoV-2 infection from 1.3% to approximately1.287% is of meaningful clinical benefit to study subjects and the American People? Yes or No. Please explain your answer.
2. You claim there were ‘no new substantive concerns regarding safety and efficacy of BNT162b, since the June 10, 2021, VRBPAC meeting. Why did you not find it concerning that in a triple cohort of over 75,000 COVID-19 patients, 1% of subjects injected with the Pfizer-BioNTech vaccine experienced serious adverse events?
3. Why did the FDA not commission the full data from the Mayo Clinical set by emailing venky@nference.net to review and analyze before providing BLA approval?
4. the Mayo Clinic data, do you believe that a 86% or 76% chance in reducing the risk of the rate of SARS-CoV-2 infection from 1.5% to approximately 1.48% for up to two weeks post second dose is of meaningful clinical benefit to study subjects and the American People? Yes or No. Please explain your answer.
5. As an American citizen and taxpayer, do you believe the clinical benefit of reducing the risk of rate of infection by 1% for a disease that has an extremely low infection rate of 1.3% and less than a 0.01% mortality rate for healthy individuals, is worth the approximate $100 billion dollars spent to bring these ‘COVID-19’ vaccines to market, and 100’s of billions of dollars more for boosters and vaccine surveillance systems, at the expense of other health care initiatives that can promote physical fitness, nutrition, emotional wellness, healthy sleeping patterns, prevent drug and alcohol addiction, prevent, obesity, reduce bullying and teach healthy online behaviors, and other programs that can help Americans and their children lead more fulfilling, joyful, and successful lives? Yes or No. Please explain your answer.
6. Is the over $100 billion spent and continuing to be spent on the COVID-19 vaccines money well-invested in the best interests of the American people’ and their children’s physical and emotional health and well-being? Yes or No. Please explain your answer.
Knowing that the standard in medical information communications is to presume that the understanding of a product claim or educational message is by an individual with no medical or scientific expertise with a 6th grade reading level, you are fully aware that when a subject sees “95% effective” they understand that to mean a 95% reduction in absolute risk of infection compared to placebo or control group. It is deceptive to pretend to assume that the average American would believe “95%” effective meant a 95% chance of having 1% reduction in absolute risk of infection.
Below is one example of the CDC claiming that the Pfizer-BioNTech is 95% effective in preventing confirmed infection from, presumably, SARS-CoV-2, the virus that causes COVID-19.
VE CLAIMS - QUESTIONS
7. Why do you allow for the CDC to make false and misleading claims regarding the efficacy of the vaccines to the American people?
8. Why have you not instructed the CDC to provide an accurate statement regarding ALL of the vaccine’s absolute efficacy? According to the data filed with the FDA, they are about 1% effective in reducing the rate of infection from SARS-CoV-2, NOT 86%, 92%, 95% or 100% or whatever false and misleading numbers the CDC continues to propagate with the permission and guidance of the FDA.
VACCINE EFFECTIVENESS
In addition to measuring ‘vaccine efficacy,’ the Mayo Clinic also measured several outcomes of vaccine efficacy, including ‘COVID-19 Breakthrough Infections.” Breakthrough Infection was defined as SARS-CoV-2 confirmed by a positive PCR test occurring at least 14 days after the second dose of mRNA1273 or BNT162 (aka, ‘fully vaccinated’ subjects).
Per Tables 1 and 8 of the study, there were 21,179 fully vaccinated subjects in the Moderna (mRNA-1273) group, of which 106 experienced “COVID-19 Breakthrough Infections” = 0.5%. There were 22,064 fully vaccinated subjects in the Pfizer BioNTech (BNT162b) group, of which 220 experienced “COVID-19 Breakthrough Infections” = 1.0%.
“COVID-19 BREAKTHROUGH INFECTIONS”
Below is Table 8 of the study detailing the primary list of symptoms of ‘COVID-19 BREAKTHROUGH Infections” in fully-vaccinated individuals. The breakthrough incidence of 1% in the Pfizer group of COVID-19 cases resulted in serious illnesses such as ARD/ALD (often caused by immunogenicity or ADE), acute kidney injury/disease, anemia (aka thrombocytopenia), thrombosis, cardiac arrest, chronic fatigue syndrome (primarily caused by encephalitis and/or myelitis), heart failure (cause by LVH inflammation, aka myocarditis), disseminated intravascular coagulation, sepsis (aka MIS-C), stroke et al. This was alarming to the investigators in regards to the effectiveness of the vaccines to prevent serious infection, and potentially strengthens the need for boosters.
I don’t believe the serious illnesses stated in the Mayo Clinic’s list of “COVID-19 BREAKTHROUGH INFECTIONS” were due to the coronavirus. I believe they were serious adverse events caused by the COVID-19 ‘vaccines’ themselves. The FDA VRBPAC met on October 22, 2020, and made a list of the foreseeable serious adverse event (SAEs) outcomes from the EUA COVID-19 VACCINES. Although I am not a doctor or a scientist, I am literate, and the Mayo Clinic “COVID-19 INFECTIONS” list matches up verbatim (with some minor wordsmithing) to the list of foreseeable SAEs from COVID-19 vaccines per the VRBPAC Meeting.
Dr. Woodcock, I do not believe you to be delusional or lack the intelligence to understand that the clinical evidence overwhelmingly proves that the Pfizer-BioNTech and Moderna COVID-19 vaccines cause these serious adverse events, at a minimum rate of 1% within a period of days or a just few months post second-dose. It’s important to note, that Table 8 does not include the “Breakthrough Infections” or hospitalizations after 1-dose.
To date, there have been a total of approximately 645,000 Americans who have died with COVID-19 out of a population of approximately 331.5mm. About 0.2% Americans have died with COVDI-19 over the last 18 months. Generously hedging, about 96,750 died from COVID-19, or about 0.03% over the last 18 months.
BREAKTHROUGH INFECTIONS/SAEs – QUESTIONS:
1. Why does the list of illnesses in the Mayo Clinic Study defined as “Breakthrough COVID-19 Infections” match nearly verbatim to the list of foreseeable serious adverse events created by the FDA VRBPAC of possible serious adverse events?
2. Also, please explain the risk benefit ratio of being injected with the Pfizer- BioNTech COVID-19 Vaccine - with a safety risk of 1% for an SAE, and an efficacy benefit of a 1% reduction in risk of infection from a disease with a 1.3% infection rate and 0.01% mortality rate or lower for the majority of Americans. Please show your analysis.
3. To better understand the tyrannical demand for vaccine mandates in our schools, military, and workplaces, please explain the risk-benefit analysis for healthy young adults and teens to get the Pfizer BioNTech vaccine. Please show your analysis.
4. Based on your analysis, if young healthy adults, teens, and children have less than a 0.004% of death from COVID-19, why do they need to be vaccinated?
FDA BLA RESPONSE: ¶1:3
“The information provided in the biologics license application met all the FDA standards for quality, safety, and effectiveness for licensure.” – Janet Woodcock, MD
FDA CLAIM: “INFORMATION MET ALL FDA STANDARDS FOR QUALITY, SAFETY, AND EFFECTIVENESS”
Although you state the data submitted for BLA approval met all the FDA standards for quality, safety, and effectiveness for license, per 21 USC Sec. 312.44 IND TERMINATION, your statement is disingenuous. The COVID-19 EUA Vaccines never should have progressed passed Phase 1 human trials, never mind Phase 2 or 3 trials, and onto full licensure approval. If you believe I am mistaken, please provide your counterargument with documented clinical evidence to substantiate your stance.
21 U.S.C Sec. 312.44 TERMINATION
a) General. This section describes the procedures under which FDA may terminate an IND. If an IND is terminated, the sponsor shall end all clinical investigations conducted under the IND and recall or otherwise provide for the disposition of all unused supplies of the drug. A termination action may be based on deficiencies in the IND or in the conduct of an investigation under an IND. Except as provided in paragraph (d) of this section, a termination shall be preceded by a proposal to terminate by FDA and an opportunity for the sponsor to respond. FDA will, in general, only initiate an action under this section after first attempting to resolve differences informally or, when appropriate, through the clinical hold procedures described in § 312.42.
(b)Grounds for termination - (1) Phase 1. FDA may propose to terminate an IND during Phase 1 if it finds that:
(i) Human subjects would be exposed to an unreasonable and significant risk of illness or injury.
· Per the FDA’s own VRBPAC Powerpoint presentation, slide 16, dated October 22, 2020, the FDA lists anticipated serious adverse events that can result in permanent damage, disability, and death.
· Per pages 67-68, Sec. 8.3.5.1. Exposure During Pregnancy (EDP): Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines, and EDP occurs if:
o A female family member or healthcare provider reports that she is pregnant after having been exposed to the study intervention by inhalation or skin contact.
o A male family member or healthcare provider who has been exposed to the study intervention by inhalation or skin contact then exposes his female partner prior to or around conception.
o LAST PARAGRAPH Page 68: Neonatal deaths that occur within 1 month of birth should be reported, without regard to causality, as SAEs. In addition, infant deaths as related or possibly related to exposure to the study intervention.
· Per pages 69, Sec. 8.3.5.2. Exposure During Breastfeeding: Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines, An exposure during breastfeeding occurs if:
o A female participant is found to be breastfeeding while receiving or after discontinuing study intervention.
o A female is found to be breastfeeding while being exposed or having been exposed to study intervention (i.e. environmental exposure)….female family member or healthcare provider who reports that she is breastfeeding after having been exposed to the study intervention by inhalation or skin contact.
The FDA knew of the high-risk of birth defects and deaths to unborn and newborn babies, and still proceeded with the Pfizer-BioNTech COVID-19 trials and EUA.
Below is an example the tragic death of a perfectly healthy 5-month- old baby boy as a result of the FDAs own willful violation of 21 USC 312.44.
QUESTIONS:
· Based on the risk of transmission from injected subjects to pregnant mothers alone, why did the FDA not stop the trials of Pfizer-BioNTech COVID-19 Vaccines?
· Based on the known risks of harm and death to newborns and infants through shedding from injected subjects, why did the FDA not stop the trials of Pfizer-BioNTech COVID-19 Vaccines?
· Why did the FDA not warn pregnant mothers and parents of newborns and infants of these risks?
(ii) The IND does not contain sufficient information required under § 312.23 to assess the safety to subjects of the clinical investigations.
· The majority of animal studies were never initiated or incomplete. Please provide a summary and full data sets of all animal studies completed to date.
· Among other animal studies, the FDA never had the manufacturer initiate or complete progeny and shedding studies per the FDA’s own August 2015 Guidance Document, “Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products,” resulting in tens of millions of Americans producing harmful virion particles, i.e. spike proteins, while putting others at high risk of infection through shedding. Pregnant women are especially at high-risk of teratogenic risks and infants are at high-risk for serious illness from vaccinated family members and friends.
· The risk of harm from shedding to pregnant women and to their infants is self-evident in the FDA’s own BLA lettered dated August 23, 2021, requiring to assess such risks as stated above per page 9, item 10. “Pfizer BioNTech COVID-19 Exposure During Pregnancy: A Non-Interventional Post-Approval Safety Study of Pregnancy and Infant Outcomes in the Organization of Teratology Information Specialists (OTIS)/MotherToBaby Pregnancy Registry.”
· Thousands of pregnant mothers experienced miscarriages and many parents suffered the death of their newborns or infants because of the FDAs own willful violation of 21 USC 312.44.
· “INSERT VAERS DATA OF MISCARRIAGES AND NEWBORN DEATH”
QUESTIONS:
· Why did the FDA not require the CDC, NIH, MSM, employers, local and federal governments, and school boards to warn pregnant mothers and parents of newborns and infants of these risks?
· Why did the FDA allow these deaths to happen with zero intervention and risk mitigation strategies?
· Can you specifically address your knowledge of and determination of the risks of immunogenicity (antibody-dependent-enhancement), teratogenicity, cardiac serious adverse events, cardiovascular serious adverse events, central nervous system serious adverse events, pulmonary serious adverse events, risks of progeny and viral shedding compared to efficacy benefits per the SARS-Cov-2 and/or COVID-19 IRR, IFR, CFR, and hospitalization risk by age group and co-morbidities?
(iii) The methods, facilities, and controls used for the manufacturing, processing, and packing of the investigational drug are inadequate to establish and maintain appropriate standards of identity, strength, quality, and purity as needed for subject safety.
· Per the EUA regulations, the majority of cGMP (consumer good manufacturing practices) were abandoned including inspection of manufacturing facilities, quality control testing labs, and confirmation of final contents and quality of final vaccine vial ingredients.
· Per the FDA’s own BLA letter, final contents of the vaccine vials must be confirmed by September 6, 2021, along with the finalization of the labeling, ingredients, side effects, and serious adverse events.
QUESTION: What CONTROL PROTOCOLS WERE ENFORCED BY THE FDA TO ENSURE appropriate standards of identity, strength, quality, and purity as needed for SUBJECT SAFETY who were injected under the EUA?
(iv) The clinical investigations are being conducted in a manner substantially different than that described in the protocols submitted in the IND.
· Per page 9 of the IND, Rationale: Pfizer IND, Phase 1/2/3, RNA-Based COVID-19 Vaccines, the document states:
o The 2 SARS-CoV-2 vaccine candidates that will be tested in this study are therefore:
§ BNT162b1 (variant RBP020.3): a modRNA encoding the RBD;
§ BNT162b2 (variant RBP020.2): a modRNA encoding P2 S.
o All candidates are formulated in the same lipid nanoparticle (LNP) composition.
o This study is intended to investigate the safety, immunogenicity, and efficacy of these prophylactic BNT162 vaccines against COVID-19.
Dr. Fauci, and numerous healthcare leaders and influencers, informed the American people that the mRNA vaccines would produce the spike protein of the SARS-CoV-2, the virus that causes COVID-19, and then their bodies would produce neutralizing antibodies. However, the BNT162b2 variation of the mRNA vaccine does not produce the SARS-CoV-2 spike protein. BNT162b2 produces the SP-2 spike protein which had 2 prolines added to it, changing the spike protein’s conformation (shape) and receptor binding domains. According to a February 19, 2020, publication in Science, the SP-2 spike protein produced by the mRNA vaccines binds beautifully with the ACE-2 receptor sites in the heart, lungs, and kidneys, resulting in inflammation, disease, and/or death, BUT has nearly zero binding activity to the neutralizing antibodies of the SARS-CoV-2 spike protein.
QUESTIONS:
· Per the data presented in the Feb 19, 2020 article in Science, the chimeric coronavirus-based mRNA in BNT162b produces SP-2, a spike protein that is highly potent at inducing inflammation and disease but has zero binding activity to the neutralizing antibodies of the SARS-CoV-2 spike protein. In your expert opinion, how does SP-2 produce immune protection from SARS-CoV-2?
· The SP-2 conformation and mechanism-of-action data in Science was available to you months prior to IND approval and EUA authorization of BNT162b. What is the clinical justification for your approval of the IND and EUA for BNT162b?
Page 1, ¶4 of the August 23, 2021 FDA Approval letter states : “The review of this product was associated with the following National Clinical Trial (NCT) numbers: NCT04368728 and NCT04380701.”
· NCT04368728 - https://clinicaltrials.gov/ct2/show/NCT04368728
· NCT04380701 - https://clinicaltrials.gov/ct2/show/NCT04368728
Per the NCT Study descriptions filed on April 30, 2020, a third version of the Pfizer-BioNTech COVID-19 Vaccines known as BNT162b2VOC (or BNT162bSA) would be tested on Phase 3 trial participants for the South African variant.
In January of 2021, the World Health Organization, published articles discussing the emergence of Coronavirus variants, including ones from South Africa, but no South African variant had been clearly identified in January of 2021.
QUESTIONS:
· In April of 2020, why did you approve human research on American citizens for a chimeric-viral based mRNA genetic agent (BNT162bSA) that had NEVER been TESTED IN ANIMALS and to purportedly prevent infection from a virus that didn’t even exist yet?
· Why did you willfully violate 21 U.S.C Sec. 312.44 (iv)? Why did you not immediately terminate the request to even begin trials of this biological agent?
Per page the August 23rd BLA Letter and the FDA EUA Authorization Letters, the FDA authorization and approval of the Pfizer BioNTech COVD-19 vaccines are associated with the product(s) used in the IND/NCT trials which include the following mRNA versions and varying dosages;
1. BNT162b1: 10µg dose: mRNA encodes RBD – receptor binding domains
2. BNT162b2: 10µg dose: mRNA encodes PS 2 - spike protein
3. BNT162b1: 20µg dose: mRNA encodes RBD – receptor binding domains
4. BNT162b2: 20µg dose: mRNA encodes PS 2 - spike protein
5. BNT162b1: 30µg dose: mRNA encodes RBD – receptor binding domains
6. BNT162b2: 30µg dose: mRNA encodes PS 2 - spike protein
7. BNT162b2SA: 30µg dose: mRNA encodes South African spike protein
8. BNT162b1: 100µg dose: mRNA encodes RBD – receptor binding domains
9. BNT162b2: 5µg booster dose: mRNA encodes PS 2 - spike protein
10. BNT162b2: 10µg booster dose: mRNA encodes PS 2 - spike protein
11. BNT162b2: 30µg booster dose: mRNA encodes PS 2 - spike protein
12. Other: Placebo
QUESTIONS:
· What is the mechanism of action of the mRNA encoding receptor binding domains of 162BNTb1 in preventing infection from SARS-CoV-2, the virus that causes COVID-19?
· Are there any known pathogenic attributes of mRNA BNT162b1?
The American people are under the impression that they if they received the EUA Pfizer vaccine or will be receiving the COMIRNATY BLA APPROVED vaccine, both vaccines are the Phase 3 BNT162b2 version at a 30mcg dose per dosage. Based on the confusing wording of the EUA and BLA letters, this is unclear. Please clarify.
QUESTIONS:
· What mRNA version(s) were in the EUA Pfizer-BioNTech vaccines administered to the American people? At what dosage(s)?
· What quality control measures were in place to ensure the BNT162b2 vaccines manufactured under the EUA (or other versions), prior to COMIRNATY BLA approval, were consistent with the ingredients listed in the Phase 3 NDA filed on November 20, 2020?
· What formulation(s) of BNT162b is/are in the FDA licensed COMIRNATY?
· What dosage(s) of mRNA is/are in COMIRNATY?
(v) The drug is being promoted or distributed for commercial purposes not justified by the requirements of the investigation or permitted by § 312.7.
· Section 312.7 is related to investigators submitted unreliable data. The Americna people have 85% of the investigators and their subjects were outside the U.S., in Brazil, Argentina, South Africa, Germany and Turkey.
QUESTIONS:
· What independent US monitoring boards were in place to ensuring the reliability of the data submitted?
· Which investigators and/or their clinical research sites had financial ties to the manufacturers (Pfizer-BioNTech) and any non-US government parties or organizations? What were those financial ties?
In regards to unreliable data, pg. 24, Table 7. of the Pfizer Phase 3, November 20, 2020, EUA submission, there were 169 placebo subjects who presented with COVID-19 symptoms confirmed with a positive PCR test, and only 9 subjects in the BNT162b group, resulting in ‘statistically significant’ ‘vaccine efficacy.’
Interestingly, pg. 41,¶2:2 of the Pfizer Phase 3, November 20, 2020, EUA submission, clearly states, “Suspected COVID-19 cases that occurred within 7 days after any vaccination were 409 in the vaccine group vs. 287 in the placebo group.”
If this data was accurately and ethically accounted for, the primary ‘vaccine efficacy’ endpoint for the Pfizer-BioNTech COVID-19 Vaccine would have shown zero statistical significance.
Page 41,¶2:3 of the Pfizer document goes on to state, “It is possible that the imbalance in suspected COVID-19 cases occurring in the 7 days postvaccination represents vaccine reactogenicity with symptoms that overlap with those of COVID-19. Overall though, these data do not raise a concern that protocol-specified reporting of suspected, but unconfirmed COVID-19 cases could have masked clinically significant adverse events that would not have otherwise been detected.”
· Per § 312.7.e) If the FDA Commissioner determines, after the unreliable data submitted by the investigator are eliminated from consideration, that the continued approval of the product for which the data were submitted cannot be justified, the FDA Commissioner will proceed to withdraw the approval of the product in accordance with the applicable provisions of the relevant statutes.
In my opinion, your gross negligence in ignoring these blatant violations of § 312.7.e can only be explained by willful misconduct. However, I will provide you with an opportunity to respond, as I believe the American people have a right to know the motivations behind your decisions.
QUESTIONS:
· As the FDA Commissioner, why did you not point out that the data provided for the Pfizer-BioNTech is unreliable per the sponsor’s own admission?
· As the FDA Commissioner, why did you allow for the sponsor to make the contradictory claims that the serious illnesses that manifested within 1-week of the second dose of BNTA162b in an additional 409 subjects WERE NEITHER COVID-19 CASES nor were they SERIOUS ADVERSE EVENTS?
(vi) The IND, or any amendment or report to the IND, contains an untrue statement of a material fact or omits material information required by this part.
· UNTRUE STATEMENT OF MATERIAL FACT: The BNT162b products used in the trials do not meet the clinical definition, per the FDA, or legal definition, per the USPTO, of a vaccine. The BNT162b products meet the definition of a “Viral-Based Gene Therapy”
· Per the FDA’s own August 2015 Guidance Document, “Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products, “Gene therapy products are all products that mediate their effects by transcription and/or translation of transferred genetic material and/or by integrating into the host genome and that are administered as nucleic acids (mRNA), viruses, or genetically engineered microorganisms.”
(vii) The sponsor fails promptly to investigate and inform the Food and Drug Administration and all investigators of serious and unexpected adverse experiences in accordance with § 312.32 or fails to make any other report required under this part.
· The VAERS data was willfully and unconscionably ignored by the FDA, CDC, NIH, and Pfizer. Furthermore, there are tens of thousands, if not hundreds of thousands, of testimonials from victims, family members of victims, and healthcare providers of the EUA COVID-19 ‘vaccine’ experiment, that were ignored or dismissed by the government agencies involved and their collaborators in the mainstream media, social media, healthcare, employer, and education markets.
FDA BLA RESPONSE: ¶3:2-5
“…the existence of reports in VAERS does not mean that a cause-and-effect relationship has been established. FDA has found that many reports in VAERS are not documentation that a vaccine caused the reported event...Reports of death after COVID-19 vaccinations that are found to be related, or even possibly related, to vaccination with COVID-19 vaccines have been extremely rare.” – Janet Woodcock, MD
Dr. Woodcock, your written statement regarding your opinion or facts that deaths and serious adverse events in VAERS related to the COVID-19 Vaccines is disingenuous.
Per page 6, ¶3 of the August 23, 2021, FDA-Pfizer BioNTech BLA Approval Letter, the FDA states;
“We have determined that an analysis of spontaneous postmarketing adverse events reported under section 505(k)(1) of the FDCA will not be sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis”
The above paragraph specifically refers to the data collected in VAERS, and that that data is sufficient to establish a correlation between the Pfizer BioNTech Covid-19 ‘vaccine; and myocarditis and pericarditis, enough so to merit conducting several postmarketing Phase 4 studies to determine the degree, severity, and sequelae of cardiac disease cause by the ‘vaccines.’
Furthermore, per the FDA/CBER October 2019 document, Postmarketing Studies and Clinical Trials—Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act Guidance for Industry, clearly states:
The determination of the …. VAERS’s sufficiency to meet the purposes described in section 505(o)(3)(B) of the FD&C Act is based, in the context of the serious risk related to the use of the particular drug, on considerations of the strengths and limitations of adverse events reports as information sources and on the particular data characteristics of the …. VAERS systems. Therefore, FDA determines the ….VAERS’s sufficiency for the purposes of whether to require a postmarketing study or clinical trial on a case-by-case basis for each serious risk.
Your own administration’s documents and regulations clearly state that postmarketing safety studies are merited when there is a correlation between a serious adverse event and an investigational drug (vaccine).
QUESTIONS:
· Why do you disagree with your own administration’s guidance?
· If the reasons for conducting the postmarket safety studies for myocarditis and pericarditis were not based on the real-world evidence of vaccine-induced cardiac disease and death per the VAERS database, in your expert opinion, what were these postmarket studies based on?
Dr. Woodcock, I think all Americans would agree that there has been a great deal of confusion regarding the accurate information regarding these vaccines, resulting in heightened concerns regarding their safety. Per Sec. 3024 of 21st Century CARES Act, the American people’s right to informed consent under an EUA drug or vaccine is not required.
· (Sec. 3024) Clinical testing of investigational medical devices and drugs no longer requires the informed consent of the subjects if the testing poses no more than minimal risk to the subjects and includes safeguards.
There are too many blatant violations of human rights that Sec. 3024 enable, including crimes against humanity, however in the interest of productivity and transparency I believe after 18-months of nonsensical mandates including lockdowns, mask wearing, social distancing, and now the dangerously aggressive emergence of vaccine mandates, at a minimum, the American people have a right to know what is in the Pfizer BioNTech EUA and BLA COVID-19 ‘vaccines.’
On June 7, 2021, AGC Biologics announced manufacturing agreements to produce plasmid DNA (pDNA) for BioNTech, the manufacturing partner of the Pfizer-BioNTech.
QUESTIONS:
· What is the difference between pDNA and rDNA?
· What is its role of pDNA in the mRNA vaccines?
Per the Phase 3 filings, draft package insert, both PfizerBioNtech BNT162b and Moderna mRNA-1272 contains lipid nanoparticles (LNP).
Per the world patent that covers the mRNA LNP vaccines, WO 2020/160397 A1, issued to Moderna on August 6, 2020, covering the ‘art’ or intellectual property in the Pfizer BNT162 and Moderna mRNA-1273 vaccines, Sec. 0002 states:
· The present disclosure provides novel methods of producing nucleic acid (“mRNA”) lipid nanoparticle (LNP) formulations…and the related therapeutic or diagnostic uses.
A diagnostic is a not a vaccine. A diagnostic is a medical device.
Under TITLE 21 of the FD&C Act, Sec. 814.9: Confidentiality of data and information of pre-market approval (PMA) of medical devices, states;
(b) The existence of a PMA file may not be disclosed by FDA before an approval order is issued to the applicant unless it previously has been publicly disclosed or acknowledged.
QUESTIONS:
· What is the medical device(s) in the Pfizer BioNTech ‘vaccines’?
· What materials are the devices comprised of?
· What are the medical devices functionalities in regards to how they transmit and exchange data?
· What are the associated risks of permanent harm, disability, or death to human subjects who were injected with these medical devices?
In summary, based on the information available to, submitted to, and published by the FDA, it appears that the COVID-19 vaccines provide no clinical benefit and can only harm, disable, and kill American people.
If there is clinical and scientific evidence that clearly disproves the above stated claims, I urge you to release that data to the American people immediately. You must disclose any and all information regarding the adverse events of these vaccines and their components immediately to the American people.
Keep reading with a 7-day free trial
Subscribe to The Kingston Report to keep reading this post and get 7 days of free access to the full post archives.